42 label all bonds in so2
Che 140 Ch 9, 10, 11, 12 Study Guide Flashcards - Quizlet Label each variable in the ideal gas law with the property it represents. ... The following bonds, C-H and C-C, are _____ bonds. 4. The following bonds, C-F, C-O, and O-H, are _____ bonds. 1. Electronegativity ... both SO2 and H2S. One would expect that since CO is polar, it has a higher boiling point than C6H14. quizlet.com › 195082504 › chem-flash-cardschem Flashcards - Quizlet 1. When SO2 is mixed with O2 in a container, the initial rate of the forward reaction (production of SO3) is faster than the initial rate of the reverse reaction (production of SO2). 2. As SO2 is used up and SO3 accumulates, the rate of the forward reaction increases and the rate of the reverse reaction decreases. 3.
quizlet.com › 304963035 › ch18-mastering-chemistryCH18 - Mastering Chemistry - Alaa Hashim Flashcards - Quizlet The average bond enthalpies of the C - F and C - Cl bonds are 485 kJ /mol and 328 kJ / mol, respectively. What is the maximum wavelength that a photon can possess and still have sufficient energy to break the C - F and C - Cl bonds, respectively?

Label all bonds in so2
Solved Label all bonds in SO2. Label the diagram by dragging - Chegg Transcribed image text: Label all bonds in SO2. Label the diagram by dragging the labels to the appropriate targets. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o: S(sp) - O() : S(p) - (p) O: S(p) - (sp) Lone pair in p orbital T: S(sp?) assign.co.za › current-vacanciesCurrent Vacancies – Assign Services Job functions: Generate leads to sell and buy property Counsel clients on market conditions, prices, and bonds Develop competitive market price analysis’s by comparing properties Prepare and complete all required documentation pertaining to the sale of the property Show properties to potential buyers Present purchase offers to seller’s ... EOF
Label all bonds in so2. Success Essays - Assisting students with assignments online We double-check all the assignments for plagiarism and send you only original essays. Chat With Your Writer. Communicate directly with your writer anytime regarding assignment details, edit requests, etc. Affordable Prices. We offer the lowest prices per page in the industry, with an average of $7 per page. CHEM: Chapter 10 Flashcards - Quizlet Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3. Valence Bond Theory 76. Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the p2p orbitals lie at higher energy than the s2p, draw MO energy ... Answered: In the sketch of the structure of SO2… | bartleby Transcribed Image Text: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S (p) - O (p) Lone pair in p orbital Lone pair in sp? orbital o : S (p) - 0 (sp²) т: S (p) — О (p) T: S (sp²) - O (p) r: S (sp²) - O (p ... › questions-and-answers › a-weatherAnswered: A weather balloon calibrated at 0.00… | bartleby A balloon that is 100.21 L at 21 C and 0.981 atm is released and just barely clears the top of Mount crumpet in British Columbia. If the final volume of the balloon is 144.53 L at a temperature of 5.24 C, what is the pressure experienced by the balloon as it clears Mount Crumpet?
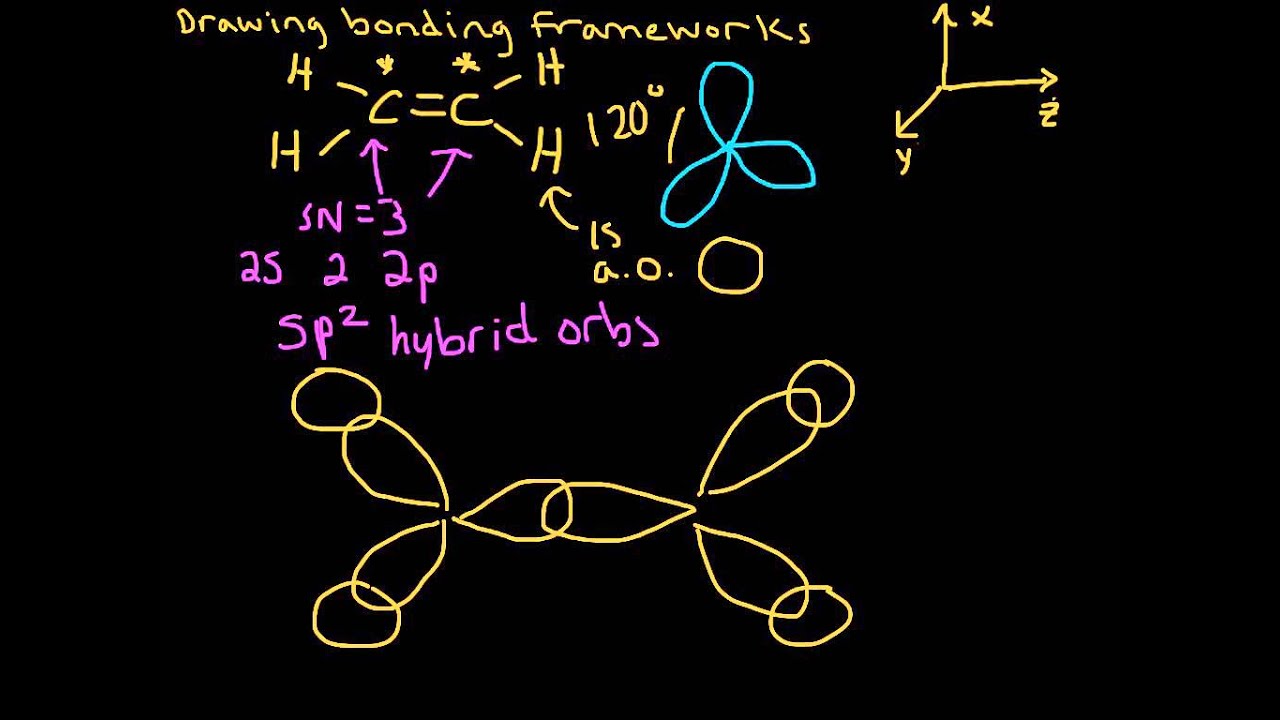
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3. Answer (a) See solution (b) See solution (c) See solution (d) See solution. View Answer. Related Courses. Chemistry 101. Chemistry. Chapter 6. inorganic chemistry - How does SO2 have 2 π bonds? - Chemistry Stack ... 1 Answer. Yes, you are right. One electron from 3p orbital is unpaired and excited to a 3d orbital. The hybridization of sulfur atom is sp2 hence a lone pair and two bond pairs (due to sigma bonding) reside in these hybrid orbitals. The unpaired electrons are 3p and 3d hybridized orbitals are used in pi bonding with oxygen's unhybridized 2p ... researchtweet.com › orbital-hybridization-sp1-sp2Orbital Hybridization: sp1, sp2, and sp3 ... - Research Tweet Aug 01, 2021 · sp Hybridization: When Carbon is bound to two other atoms with the help of two double bonds or one single and one triple bond. Example: Hybridization of CO2. sp2 Hybridization: When carbon atom bonding takes place between 1 s-orbital with two p orbitals then the formation of two single bonds and one double bond between three atoms takes place. SO2 bond sigma and pi bond - CHEMISTRY COMMUNITY In SO2, the central atom S is double bonded to each O. There are two double bonds in the structure. You know that there can only be one sigma bond in every bond. So in each double bond, there will be a sigma bond and a pi bond. In total, there will be two sigma bonds and two pi bonds. Another thing to keep in mind is that a sigma bond is ...
Journal of the American Chemical Society | Vol 143, No 48 Effect of strong intermolecular interactions induced by unique short intermolecular Se–Se and P–Se contacts in 2D inorganic molecular crystal α-P4Se3 nanoflakes is reported. We revealed the physical picture and origin of the unusual short interatomic contacts, as well. This work sheds new light on the intermolecular interactions in 2D inorganic molecular crystals. View the article. What orbitals are used to form the 10 sigma bonds in ... - Brainly.com All the bonds in the compound is single bond(-bond) that is they are formed by head on collision of the orbitals. The structure of the compound is shown in the image. The Carbon-Hydrogen bond is formed by overlapping of s-orbital of hydrogen to p-orbital of carbon. Solved In the sketch of the structure of SO2 label all | Chegg.com Expert Answer. Answer …. View the full answer. Transcribed image text: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Reset Help T: S (p) - O (p) o : S (sp²) - O (p) : S (p) - O (p) o : S (p) - O (sp²) T: S (sp² ... CHEM 123 Sapling Learning Chapter 11 Flashcards - Quizlet A σ bond is present in all covalent bonds. A π bond results from the side‑on overlap of orbitals. ... Therefore, SO2 is polar. CH2Cl2 has four bonds in a tetrahedral shape, but the C−H bonds have a different polarity than the C−Cl bonds. ... (also known as diimine), is commonly used as a reagent for organic syntheses. Label each image ...
Sulfor dioxide: Lewis dot structure for SO2 (video) - Khan Academy The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
Answered: Suppose that a market has the following… | bartleby Now suppose an excise tax is imposed on X such that the new supply equation is P = 4 +.4Q. How much tax revenue will this excise tax yield the government? Graph the curves, and label the area of the graph that represents the tax collection “TC” and the area that represents the effificiency loss of the tax “EL.”
What is the hybridisation of sulphur dioxide? - Quora Answer (1 of 8): Sulphur dioxide is SO2 The central sulphur atom is bonded to two oxygen atoms. The structure is like this: O=S=O The sulphur atom forms one sigma and one pi bond with each oxygen atom and has one lone pair. Sulphur in its ground state has first two shells completely filled and...
Essay Fountain - Custom Essay Writing Service - 24/7 Professional … We will take care of all your assignment needs. We are a leading online assignment help service provider. We provide assignment help in over 80 subjects. You can request for any type of assignment help from our highly qualified professional writers. All your academic needs will be taken care of as early as you need them. Place an Order
Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B(p) - F(p) Empty p orbital Lone pair in p orbital B B(sp²) - F(p) в : В(8) — F(p) o : B(p) - F(p) Empty sp? orbital
Inorganic Chemistry 4th edition, Catherine Housecroft Inorganic Chemistry 4th edition, Catherine Housecroft
Solved Label all bonds in SO2. The hybridization of the S - Chegg This problem has been solved! See the answer. See the answer See the answer done loading. Label all bonds in SO2. The hybridization of the S atom in SO2 is sp^2. Label all bonds in NF3. Hybridization of N atom in NF3 is sp^3. Show transcribed image text. Expert Answer.
What is Sodium Metabisulfite (E223) in food? Uses and Safety May 18, 2020 · White crystals or crystalline powder. Slowly oxidized to Na2SO4 (sodium sulfate) and release sulfur dioxide (SO2) gas if exposed to air and moisture. SO2 is also released by the reaction with acid. Solubility. Soluble in water and its water solubility increases with temperature, 54g/100ml at 20°C and 81.7g/100ml at 100°C.
What is the lewis structure for SO_2? | Socratic Now count the valence electrons you actually have available. 1 S + 2 O = 1×6 + 2×6 = 18. The trial structure has two extra electrons. 6. Draw a new trial structure, this time inserting one double bond for each extra pair of electrons: O=S-O and O-S=O. 7. As before, add valence electrons to give each atom an octet: 8.




Post a Comment for "42 label all bonds in so2"